The United States hereditary angioedema (HAE) therapeutics market is poised for significant growth in the coming decade. With a projected CAGR of 8.20% during the forecast period of 2025-2034, the market is driven by continuous advancements in novel therapies, a favorable regulatory environment, and increasing patient awareness surrounding hereditary angioedema (HAE) diagnosis and treatment options. In this blog, we will explore the market size, key trends, growth drivers, and key players in the U.S. HAE therapeutics market.
United States Hereditary Angioedema Therapeutics Market Overview
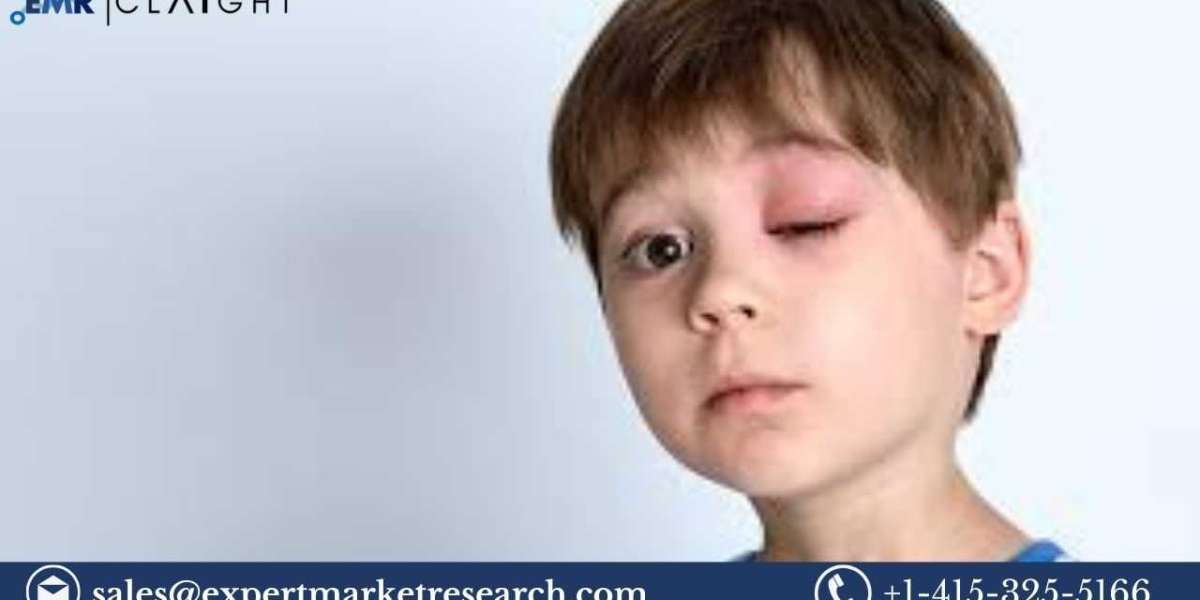
Hereditary angioedema (HAE) is a rare genetic disorder characterized by sudden episodes of severe swelling, often affecting the hands, feet, face, and airways. The condition is caused by a deficiency or dysfunction in the C1 esterase inhibitor (C1-INH), leading to uncontrolled swelling episodes. The U.S. hereditary angioedema therapeutics market focuses on developing drugs and treatments to manage and prevent these episodes, thereby improving patients' quality of life.
The market is benefiting from an increasing understanding of the disease, ongoing research into novel treatments, and enhanced patient access to therapies due to improvements in the regulatory landscape. With a growing patient population and better awareness of HAE, the demand for targeted therapeutics is expected to increase significantly in the coming years.
United States Hereditary Angioedema Therapeutics Market Size
The U.S. hereditary angioedema therapeutics market is expected to grow at a CAGR of 8.20% from 2025 to 2034, reaching a substantial market value by 2034. The market is currently valued at a notable amount, and with advancements in drug development, increasing healthcare access, and a better understanding of the disease, the market is set to experience significant growth. By the end of the forecast period, the market is projected to reach new heights as more treatments are introduced and market penetration deepens.
United States Hereditary Angioedema Therapeutics Market Share
The hereditary angioedema therapeutics market in the U.S. is currently led by a few key players offering targeted treatments that address the underlying causes of the disease. Takeda Pharmaceutical Company Limited, BioCryst Pharmaceuticals, Inc., and Sanofi hold a dominant share of the market. These companies offer innovative therapies, such as C1 esterase inhibitors, bradykinin receptor antagonists, and plasma kallikrein inhibitors, which have been pivotal in improving the outcomes for HAE patients.
The market share is also seeing a rise in competition from emerging players, which are introducing novel treatment options that expand the therapeutic landscape for HAE.
United States Hereditary Angioedema Therapeutics Market Trends
Several significant trends are shaping the U.S. HAE therapeutics market, including:
- Development of Novel Therapies: Pharmaceutical companies are focusing on developing innovative therapies that offer targeted and personalized treatment options for HAE patients. New biologics, oral therapies, and long-acting injectables are gaining attention.
- Increase in Patient Awareness: With growing awareness about HAE diagnosis and treatment, more patients are being diagnosed, leading to an increase in the demand for treatments. Patient education programs and online platforms are helping improve awareness.
- Favorable Regulatory Environment: The regulatory environment in the U.S. has become increasingly supportive of rare disease treatments, facilitating faster approval processes for HAE therapeutics. The FDA's orphan drug designation for HAE therapies has encouraged more investment in drug development.
- Focus on Personalized Medicine: There is a growing shift toward personalized medicine in the treatment of HAE, with therapies designed to address individual patient needs and optimize treatment efficacy.
- Expansion of Long-Acting Treatments: The demand for long-acting treatments that provide extended protection from swelling episodes is growing, reducing the burden on patients and improving their quality of life.
United States Hereditary Angioedema Therapeutics Market Analysis
- The U.S. hereditary angioedema therapeutics market is experiencing steady growth due to the increasing awareness of the disease, advancements in drug development, and the expanding availability of innovative therapies. While the market is dominated by major pharmaceutical companies, several emerging biotech companies are entering the space with novel therapeutic approaches.
- The market is heavily influenced by the approval of new drugs by regulatory bodies such as the FDA and EMA. This regulatory progress, combined with the market's increasing focus on patient-centric care, is expected to drive the market forward.
- However, the market also faces challenges such as the high cost of HAE treatments, the complex regulatory pathways for new therapies, and the need for continued investment in research and development.
United States Hereditary Angioedema Therapeutics Market Segmentation
The U.S. hereditary angioedema therapeutics market can be segmented based on drug type, route of administration, and end-user.
By Drug Type:
- C1 Esterase Inhibitors (C1-INH): These are the most commonly prescribed treatments for managing HAE symptoms by replacing or supplementing the deficient protein.
- Bradykinin Receptor Antagonists: Targeting bradykinin pathways to prevent swelling episodes.
- Plasma Kallikrein Inhibitors: A newer class of drugs that inhibit the enzyme kallikrein, reducing the bradykinin-mediated swelling in HAE patients.
- Other Therapies: Includes investigational drugs and monoclonal antibodies.
By Route of Administration:
- Injectables: Intravenous and subcutaneous injections are the most common methods of administration for HAE therapies.
- Oral Therapies: Oral treatments are becoming increasingly popular due to their convenience for patients.
By End-User:
- Hospitals: A significant number of treatments are administered in hospital settings, especially during acute HAE episodes.
- Specialized Clinics: Clinics specializing in rare diseases are also growing in importance as HAE diagnosis and treatment become more widespread.
- Home Healthcare: With the rise of self-administered treatments, home healthcare is expected to see growth in the coming years.
United States Hereditary Angioedema Therapeutics Market Growth
The U.S. hereditary angioedema therapeutics market is experiencing strong growth due to several key drivers:
- Innovative Drug Development: Ongoing research and development efforts are introducing novel therapies that offer improved efficacy, safety, and convenience for patients.
- Supportive Regulatory Environment: The FDA's approval of several new treatments in recent years has encouraged the growth of the market.
- Increasing Healthcare Access: Greater healthcare access, including the coverage of rare disease treatments, is helping more patients access the therapies they need.
- Growing Patient Awareness: As awareness increases, more individuals are getting diagnosed and treated, fueling market demand.
Get a Free Sample Report with a Table of Contents
Recent Developments and Challenges in the Market
Recent Developments:
- FDA Approvals: New treatments, such as berotralstat and ecallantide, have recently received approval for HAE, expanding the range of therapeutic options for patients.
- Emerging Therapies: Companies like BioCryst Pharmaceuticals and Takeda are focusing on oral and long-acting treatments for better patient compliance and convenience.
Challenges:
- High Treatment Costs: The cost of HAE therapeutics remains a challenge for both patients and healthcare providers.
- Limited Market Education: Despite increasing awareness, there is still a need for more education regarding the diagnosis and treatment of HAE.
- Regulatory Hurdles: Developing new therapies for rare diseases can be costly and time-consuming, requiring substantial investment in research and regulatory approvals.
Key Players in the U.S. Hereditary Angioedema Therapeutics Market
Takeda Pharmaceutical Company Limited: A global leader in HAE treatments, offering therapies such as Firazyr (icatibant).
BioCryst Pharmaceuticals, Inc.: Known for its innovative treatments such as Orladeyo (berotralstat) for long-term prevention of HAE attacks.
Sanofi: Offers therapies like Kanuma for rare diseases, expanding its portfolio to treat HAE.
CSL Behring LLC: A prominent player in the development of C1-INH replacement therapies.
Pharming Group N.V.: Offers Ruconest, a recombinant C1 esterase inhibitor for acute HAE attacks.
Cipla, Inc.: Developing oral therapies for the treatment of HAE and other rare diseases.
Ionis Pharmaceuticals Inc.: Working on novel RNA-targeted therapies for HAE.
Adverum Biotechnologies, Inc.: Developing gene therapies for HAE and other genetic disorders.
Arrowhead Pharmaceuticals, Inc.: Focused on gene silencing therapies for hereditary diseases.
Pharvaris B.V.: Develops innovative oral therapies targeting bradykinin for treating hereditary angioedema attacks.
 Meet Ups
Meet Ups
 Experiences
Experiences
 Learning Center
Learning Center
 Accommodation
Accommodation
 Roomie
Roomie
 Ride
Ride
 Spread the Word
Spread the Word
 Student Bazaar
Student Bazaar
 Jobs
Jobs
 Blogs
Blogs
 About StudentInsta
About StudentInsta